| AJMPR EndNote Style |
| AJMPR Template |
| Article Sample |
| Declaration Form |
| Policies and Publication Ethics |
Asian Journal of Medical and Pharmaceutical Researches (ISSN: 2322-4789) is an peer-reviewed open access journal, aims to promote communication among clinical researchers, physicians and pharmacologists worldwide. The journal publishes the fulltext of original scientific researches, reviews, case reports and short communications, quarterly on the internet. The journal aims to improve a variety of health care practices focused on the pharmaceutical industry and related areas to maintain and restore health, improve quality of life and lifespan in human beings.... view full aims and scope
Types of contributions
Research Paper: Please provide full author information, a set of keywords and an abstract in the title page. Supplementary materials can be published if necessary. Authors are encouraged to be concise although currently there is no length limit on research paper. Short Research Communication presents a concise study, or sometimes preliminary but innovative research findings that might be less substantial than a full research paper.
Review or mini-review should be authoritative and of high interest. A minimum of two figures/illustrations should be included in the review or mini-review that should be some 3000 or 5000 words long (excluding references and figure legends). High quality reviews from leading researchers in their fields are particularly welcome.
Short Research Communication is limited to 2500 words. It should have a set of keywords and an abstract summarizing background of the work, the results and their implications. Results and Discussion Section should be combined and followed by Conclusion. Materials and Methods will remain as a separate section. The number of references is limited to 30 and the number of figures and/or tables combined is limited to
Letter: Description of novel findings that might not be suitable for a regular research paper or short research communication may be published as letter. Letter is limited to be under 500 words and 5 references. There should be not more than two figures or tables combined, and no supplementary material.
Graphical Abstract
Authors should provide a graphical abstract (a beautifully designed feature figure) to represent the paper aiming to catch the attention and interest of readers. Graphical abstract will be published online in the table of content. The graphical abstract should be colored, and kept within an area of 12 cm (width) x 6 cm (height) or with similar format. Image should have a minimum resolution of 300 dpi and line art 1200dpi.
Note: Height of the image should be no more than the width. Please avoid putting too much information into the graphical abstract as it occupies only a small space. Authors can provide the graphical abstract in the format of PDF, Word, PowerPoint, jpg, or png, after a manuscript is accepted for publication. For preparing a Professional Graphical Abstract, please click here.
Submission
Please submit your manuscript via online manuscript submission portal. Please embed all figures and tables in the manuscript to become one single file for submission. Once submission is complete, the system will generate a manuscript ID and password sent to author's contact email. For questions about submission portal please contact us via editor [at] ajmpr.science-line.com.
Supplementary information: The online submission form allows supplementary information to be submitted together with the main manuscript file and covering letter. If you have more than one supplementary files, you can submit the extra ones by email after the initial submission in the submission page.
Author guidelines are specific for each journal. Our template (.doc) can assist you by modifying your page layout, text formatting, headings, title page, image placement, and citations/references such that they agree with the guidelines of journal. If you believe your work is fully edited and prepared per journal style, you can prepare your manuscript according to AJMPR Template![]() before submission.
before submission.
Submission to the Journal is on the understanding that the article has not been previously published in any other form and is not under consideration for publication elsewhere.
Supplementary materials: Supplementary materials may include figures, tables, methods, videos, and other materials. They are available online linked to the original published article. Supplementary tables and figures should be labeled with a "S", e.g. "Table S1" and "Figure S1".The maximum file size for supplementary materials is 10MB each. Please kept the files as small possible to avoid the frustrations experienced by readers with downloading large files.
.
Presentation of the article
Main Format:
First page of the manuscripts must be properly identified by the title and the name(s) of the author(s). It should be typed in Times New Roman (font sizes: 17pt in capitalization for the title, 10pt for the section headings in the body of the text and the main text, double spaced, in A4 format with 2cm margins. All pages and lines of the main text should be numbered consecutively throughout the manuscript. The manuscript must be saved in a .doc format, (not .docx files). Abbreviations in the article title are not allowed.
Manuscripts should be arranged in the following order:
1. TITLE (brief, attractive and targeted);
2. Name(s) and Affiliation(s) of author(s) (including post code) and corresponding E-mail;
3. ABSTRACT;
4. Key words (separate by semicolons; or comma,);
5. Abbreviations (used in the manuscript);
6. INTRODUCTION;
7. MATERIALS AND METHODS;
8. RESULTS;
9. DISCUSSION;
10. CONCLUSION;
11. Acknowledgements (if there are any);
12. Declarations
13. REFERENCES;
14. Tables;
15. Figure captions;
16. Figures;
Results and Discussion can be presented jointly.
Discussion and Conclusion can be presented jointly.
Article Sections Format:
Title should be a brief phrase describing the contents of the paper. The first letter of each word in title should use upper case. The Title Page should include the author(s)'s full names and affiliations, the name of the corresponding author along with phone and e-mail information. Present address (es) of author(s) should appear as a footnote.
Abstract should be informative and completely self-explanatory, briefly present the topic, state the scope of the experiments, indicate significant data, and point out major findings and conclusions. The abstract should be 150 to 300 words in length. Complete sentences, active verbs, and the third person should be used, and the abstract should be written in the past tense. Standard nomenclature should be used and abbreviations should be avoided. No literature should be cited.
Following the abstract, about 3 to 8 key words that will provide indexing references should be listed.
Introduction should provide a clear statement of the problem, the relevant literature on the subject, and the proposed approach or solution. It should be understandable to colleagues from a broad range of scientific disciplines.
Materials and Methods should be complete enough to allow experiments to be reproduced. However, only truly new procedures should be described in detail; previously published procedures should be cited, and important modifications of published procedures should be mentioned briefly. Capitalize trade names and include the manufacturer's name and address. Subheadings should be used. Methods in general use need not be described in detail. The ethical approval for using animals in the researches should be indicated in this section with a separated title.
Results should be presented with clarity and precision. The results should be written in the past tense when describing findings in the author(s)'s experiments. Previously published findings should be written in the present tense. Results should be explained, but largely without referring to the literature. In case of the effectiveness of a particular drug or other substances as inhibitor in biological or biochemical processes, the results should be provided as IC50 (half maximal inhibitory concentration) or similar appropriate manner.
Discussion should interpret the findings in view of the results obtained in this and in past studies on this topic. State the conclusions in a few sentences at the end of the paper. Both 'Results and Discussion' and 'Discussion and Conclusion' can be presented jointly.
Declarations including Ethics, Consent to publish, Competing interests, Authors' contributions, and Availability of data and materials are necessary.
Acknowledgments of persons, grants, funds, etc should be brief.
Tables should be kept to a minimum and be designed to be as simple as possible. Tables are to be typed double-spaced throughout, including headings and footnotes. Each table should be on a separate page, numbered consecutively in Arabic numerals and supplied with a heading and a legend. Tables should be self-explanatory without reference to the text. The details of the methods used in the experiments should preferably be described in the legend instead of in the text. The same data should not be presented in both table and graph forms or repeated in the text.
Figure legends should be typed in numerical order on a separate sheet. Graphics should be prepared using applications capable of generating high resolution GIF, TIFF, JPEG or PowerPoint before pasting in the Microsoft Word manuscript file. Use Arabic numerals to designate figures and upper case letters for their parts (Figure 1). Begin each legend with a title and include sufficient description so that the figure is understandable without reading the text of the manuscript. Information given in legends should not be repeated in the text.
Declarations section - Please include declarations heading
Please ensure that the sections:
-Ethics (and consent to participate)
-Consent to publish
-Competing interests
-Authors' contributions
-Availability of data and materials
are included at the end of your manuscript in a Declarations section.
Ethics Committee Approval and Patient Consent
Experimental research involving human or animals should have been approved by author's institutional review board or ethics committee. This information can be mentioned in the manuscript including the name of the board/committee that gave the approval. Investigations involving humans will have been performed in accordance with the principles of Declaration of Helsinki. And the use of animals in experiments will have observed the Interdisciplinary Principles and Guidelines for the Use of Animals in Research, Testing, and Education by the New York Academy of Sciences, Ad Hoc Animal Research Committee.
If the manuscript contains photos or parts of photos of patients, informed consent from each patient should be obtained. Patient's identities and privacy should be carefully protected in the manuscript.
Consent to Publish
Please include a ‘Consent for publication’ section in your manuscript. If your manuscript contains any individual person’s data in any form (including individual details, images or videos), consent to publish must be obtained from that person, or in the case of children, their parent or legal guardian. All presentations of case reports must have consent to publish. You can use your institutional consent form or our consent form if you prefer. You should not send the form to us on submission, but we may request to see a copy at any stage (including after publication). If your manuscript does not contain any individual persons data, please state “Not applicable” in this section.
Authors’ Contributions
For manuscripts with more than one author, AJMPR require an Authors' Contributions section to be placed after the Competing Interests section. An 'author' is generally considered to be someone who has made substantive intellectual contributions to a published study. To qualify as an author one should 1) have made substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; 2) have been involved in drafting the manuscript or revising it critically for important intellectual content; and 3) have given final approval of the version to be published. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content. Acquisition of funding, collection of data, or general supervision of the research group, alone, does not justify authorship.
We suggest the following format (please use initials to refer to each author's contribution): AB carried out the molecular genetic studies, participated in the sequence alignment and drafted the manuscript. JY carried out the immunoassays. MT participated in the sequence alignment. ES participated in the design of the study and performed the statistical analysis. FG conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
For authors that equally participated in a study please write 'All/Both authors contributed equally to this work.' Contributors who do not meet the criteria for authorship should be listed in an acknowledgements section.
Competing Interests
Competing interests that might interfere with the objective presentation of the research findings contained in the manuscript should be declared in a paragraph heading "Competing interests" (after Acknowledgment section and before References). Examples of competing interests are ownership of stock in a company, commercial grants, board membership, etc. If there is no competing interest, please use the statement "The authors declare that they have no competing interests.".
Asian Journal of Medical and Pharmaceutical Researches adheres to the definition of authorship set up by The International Committee of Medical Journal Editors (ICMJE). According to the ICMJE authorship criteria should be based on 1) substantial contributions to conception and design of, or acquisition of data or analysis and interpretation of data, 2) drafting the article or revising it critically for important intellectual content and 3) final approval of the version to be published. Authors should meet conditions 1, 2 and 3.
It is a requirement that all authors have been accredited as appropriate upon submission of the manuscript. Contributors who do not qualify as authors should be mentioned under Acknowledgements.
Change in authorship
We do not allow any change in authorship after provisional acceptance. We cannot allow any addition, deletion or change in sequence of author name. We have this policy to prevent the fraud.
Acknowledgements
We strongly encourage you to include an Acknowledgements section between the Authors’ contributions section and Reference list. Please acknowledge anyone who contributed towards the study by making substantial contributions to conception, design, acquisition of data, or analysis and interpretation of data, or who was involved in drafting the manuscript or revising it critically for important intellectual content, but who does not meet the criteria for authorship. Please also include their source(s) of funding. Please also acknowledge anyone who contributed materials essential for the study.
Authors should obtain permission to acknowledge from all those mentioned in the Acknowledgements. Please list the source(s) of funding for the study, for each author, and for the manuscript preparation in the acknowledgements section. Authors must describe the role of the funding body, if any, in study design; in the collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Data Deposition
Nucleic acid sequences, protein sequences, and atomic coordinates should be deposited in an appropriate database in time for the accession number to be included in the published article. In computational studies where the sequence information is unacceptable for inclusion in databases because of lack of experimental validation, the sequences must be published as an additional file with the article.
References:
A reference style for EndNote may be found here.
- All references to publications made in the text should be presented in a list with their full bibliographical description. DOI number or the link of article should be added to the end of the each reference.
- In the text, a reference identified by means of an author‘s name should be followed by the date of the reference in parentheses. When there are more than two authors, only the first author‘s surename should be mentioned, followed by ’et al‘. In the event that an author cited has had two or more works published during the same year, the reference, both in the text and in the reference list, should be identified by a lower case letter like ’a‘ and ’b‘ after the date to distinguish the works.
- References in the text should be arranged chronologically (e.g. Kelebeni, 1983; Usman and Smith, 1992 and Agindotan et al., 2003). The list of references should be arranged alphabetically on author's surnames, and chronologically per author. If an author's name in the list is also mentioned with co-authors, the following order should be used: Publications of the single author, arranged according to publication dates - publications of the same author with one co-author - publications of the author with more than one co-author. Publications by the same author(s) in the same year should be listed as 1992a, l992b,etc.
- Names of authors and title of journals, published in non-latin alphabets should be transliterated in English.
- A sample of standard reference is "1th Author surname A, 2th Author surname B and 3th Author surname C (2013). Article title should be regular and 9 pt . Asian Journal of Medical and Pharmaceutical Researches, Volume No. (Issue No.): 00-00." DOI:XXX."
- Journal titles should be full in references. The titles should not be italic.
- References with more than 10 authors should list the first 10 authors followed by 'et al.' in regular form.
- The color of references in the text of article is blue. Example: (Preziosi et al., 2002; Mills et al., 2015).
-Examples (at the text):
Abayomi (2000), ----- Agindotan et al. (2003), ------ (Kelebeni, 1983), ----- (Usman and Smith, 1992), ------ (Chege, 1998; Chukwura, 1987a,b; Tijani, 1993,1995), ----- (Kumasi et al., 2001). For separating author please use "and" in cited references and "et al." should be in regular form.
.
--Examples (at References section):
A) For journal:
Amiri MS, Hosseini HA and Rajai P (2014). Preliminary investigation on phytochemical composition and anti-bacterial activity of the root of Cousinia microcarpa Boiss. Asian Journal of Medical and Pharmaceutical Researches, 4 (4): 156-159.
Spandidos A, Wang X, Wang H and Seed B (2010). PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Research, 38: D792-D799.
Gordon MP and Chandler NP (2004). Electronic apex locators. International Endodontic Journal, 37:425-37.
Kareem SK (2001). Response of albino rats to dietary level of mango cake. Journal of Agricultural Research and Development, pp 31-38.
Rapee RM and Heimberg RG (1997). A cognitive behavioral model of anxiety in social phobia. Behaviour Research and Therapy, 35: 741-765.
Spandidos DA and Wilkie NM (1984.). Malignant transformation of early passage rodent cells by a single mutated human oncogene. Nature 310: 469-475.
B) For edited symposia, special issues, etc.:
Kent G (2000). Understanding the experiences of people with disfigurements: An integration of four models of social and psychological functioning. Psychology, Health & Medicine, 5(2): 117-129.
Palmer C (2005). The social competence of children with albinism. International Congress Series, 1282: 917– 921.
C) For books:
AOAC (1990). Association of Official Analytical Chemists. Official Methods of Analysis, 15th Edition. Washington D.C. pp. 69-88.
Sadock BJ and Sadock VA (2007). Comprehensive textbook of psychiatry (8th ed). Lippincott Williams & Wilkins: Philadelphia: 609-616.
Rothman KJ, Greenland S and Lash TL (2008). Modern epidemiology Medicine. 3rd Edition. Lippincott, Williams & Wilkins. Philadelphia.
D) Conference: Skinner J, Fleener B and Rinchiuso M (2003). Examining the relationship between supervisors and subordinate feeling of empowerment with LMX as a possible moderator. 24th Annual Conference for Industrial Organizational Behavior.
E) Book: Russell, Findlay E (1983). Snake Venom Poisoning, 163, Great Neck, NY: Scholium International. ISBN 0-87936-015-1.
F) Web Site: Bhatti SA and Firkins JT (2008). http://www.ohioline.osu.edu/sc1156_27.hmtl.
All the cited papers in the text must be listed in References. All the papers in References must be cited in the text
Formulae, numbers and symbols
- Typewritten formulae are preferred. Subscripts and superscripts are important. Check disparities between zero (0) and the letter 0, and between one (1) and the letter I.
- Describe all symbols immediately after the equation in which they are first used.
- For simple fractions, use the solidus (/), e.g. 10 /38.
- Equations should be presented into parentheses on the right-hand side, in tandem.
- Levels of statistical significance which can be used without further explanations are *P < 0.05, **P < 0.01, and ***P < 0.001.
- In the English articles, a decimal point should be used instead of a decimal comma.
- Use Symbol fonts for "±"; "≤" and "≥" (avoid underline).
- In chemical formulae, valence of ions should be given, e.g. Ca2+ and CO32-, not as Ca++ or CO3.
- Numbers up to 10 should be written in the text by words. Numbers above 1000 are recommended to be given as 10 powered x.
- Greek letters should be explained in the margins with their names as follows: Αα - alpha, Ββ - beta, Γγ - gamma, Δδ - delta, Εε - epsilon, Ζζ - zeta, Ηη - eta, Θθ - theta, Ιι - iota, Κκ - kappa, Λλ - lambda, Μμ - mu, Νν - nu, Ξξ - xi, Οο - omicron, Ππ - pi, Ρρ - rho, Σσ - sigma, Ττ - tau, Υυ - ipsilon, Φφ - phi, Χχ - chi, Ψψ - psi, Ωω - omega.Please avoid using math equations in Word whenever possible, as they have to be replaced by images in xml full text.
Nomenclature and Abbreviations:
Nomenclature should follow that given in NCBI web page and Chemical Abstracts. Standard abbreviations are preferable. If a new abbreviation is used, it should be defined at its first usage. Abbreviations should be presented in one paragraph, in the format: "term: definition". Please separate the items by ";".
E.g. ANN: artificial neural network; CFS: closed form solution; ...
Abbreviations of units should conform with those shown below:
| Decilitre | dl | Kilogram | kg | |
| Milligram | mg | hours | h | |
| Micrometer | mm | Minutes | min | |
| Molar | mol/L | Mililitre | ml | |
| Percent | % | |||
Other abbreviations and symbols should follow the recommendations on units, symbols and abbreviations: in “A guide for Biological and Medical Editors and Authors (The Royal Society of Medicine London 1977)”.
Review/Decisions/Processing/Policy
Manuscripts that are judged to be of insufficient quality or unlikely to be competitive enough for publication will be returned to the authors at the initial stage. The remaining manuscripts go through a single-blind review process, and possible decisions are: accept as is, minor revision, major revision, or reject. See sample of evaluation form. Authors should submit back their revisions within 14 days in the case of minor revision, or 30 days in the case of major revision. To submit a revision please go submission page, fill out the form, mark "Revised", attach the revision (MSword) and submit when completed. Manuscripts with significant results are typically reviewed and published at the highest priority.
Plagiarism: There is a zero-tolerance policy towards plagiarism (including self-plagiarism) in our journals. Manuscripts are screened for plagiarism by Docol©c a plagiarism finding tool, before or during publication, and if found they will be rejected at any stage of processing. See sample of Docol©c-Report.
Date of issue
All accepted articles are published Quarterly (around 25th of March, June, September and December, each year) in full text on the internet.
Publication Charges
No peer-reviewing charges are required. However, there is a $85 editor fee for the processing of each primary accepted paper. Payment can be made by credit card, bank transfer, money order or check. Instruction for payment is sent during publication process as soon as manuscript is accepted. Meanwhile, this journal encourage the academic institutions in low-income countries to publish high quality scientific results, free of charges.
The Waiver policy
The submission fee will be waived for invited authors, authors of hot papers, and corresponding authors who are editorial board members of the Asian Journal of Medical and Pharmaceutical Researches. The Journal will consider requests to waive the fee for cases of financial hardship (for high quality manuscripts and upon acceptance for publication). Requests for waiver of the submission fee must be submitted via individual cover letter by the corresponding author and cosigned by an appropriate institutional official to verify that no institutional or grant funds are available for the payment of the fee. Letters including the manuscript title and manuscript ID number should be sent to: editor [at] ajmpr.science-line.com. It is expected that waiver requests will be processed and authors will be notified within two business day.
The OA policy
Asian Journal of Medical and Pharmaceutical Researches is an open access journal which means that all content is freely available without charge to the user or his/her institution. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles, or use them for any other lawful purpose, without asking prior permission from the publisher or the author. This is in accordance with the BOAI definition of Open Access.
S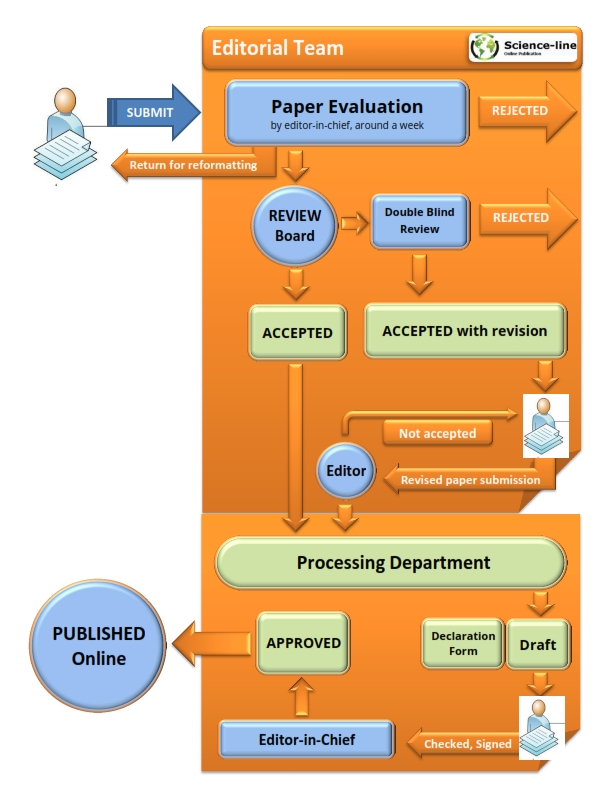 ubmission Preparation Checklist
ubmission Preparation Checklist
Authors are required to check off their submission's compliance with all of the following items, and submissions may be returned to authors that do not adhere to the following guidelines.
## The submission has not been previously published, nor is it before another journal for consideration (or an explanation has been provided in Comments to the Editor).
## The submission file is in Microsoft Word, RTF, or PDF document file format.
## Where available, URLs for the references have been provided.
## The text is single-spaced; uses a 10-point font; and all illustrations, figures, and tables are placed within the text at the appropriate points, rather than at the end.
## The text adheres to the stylistic and bibliographic requirements outlined in the Author Guidelines.
(Revised on 27 January 2019)

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
