Previous issue | Next issue | Archive
Volume 5 (2); 25 June, 2015
Immunopharmacological Activity of Zingiber officinale on Human Peripheral Blood Mononuclear Cells.
Gupta A and Chaphalkar SR. 2015.
Asian J. Med. Pharm. Res., 5(2): 13-17, 2015.; pii:S2252043015000003-5
Abstract
There are number of medicinal plant products which have been traditionally used in India including various other subcontinents for various kinds of ailments or diseases. In spite of these treatments, there is an urgent need for those substances which showed anti-inflammatory as well as anti-viral activity. To achieve this objective, our group focused on the rhizome portion of Zingiber officinale was screened for anti-inflammatory activity against specific antigen (hepatitis B vaccine, HBsAg) and also observed its antiviral activity against new castle disease virus (NDV) in human peripheral blood mononuclear cells (PBMC). The results showed that the aqueous rhizome extract of Zingiber officinale showed remarkably decline in the number of CD14 monocyte count with exposure of HBsAg and NDV as compared to control group. In addition, this extract also showed drastically reduced in the level of antigen specific HBsAg and NDV proliferation at higher doses as compared to control. NDV and HBsAg used as standard for these studies and the results showed that there is drastically increased in CD14 monocytes count and proliferation assay as compared to control. Overall, the results showed that the aqueous rhizome extract of Zingiber officinale showed anti-inflammatory as well as anti-viral activity.
Keywords: Zingiber officinale, anti-inflammatory, anti-viral, NDV
[Full text-PDF] [XML]
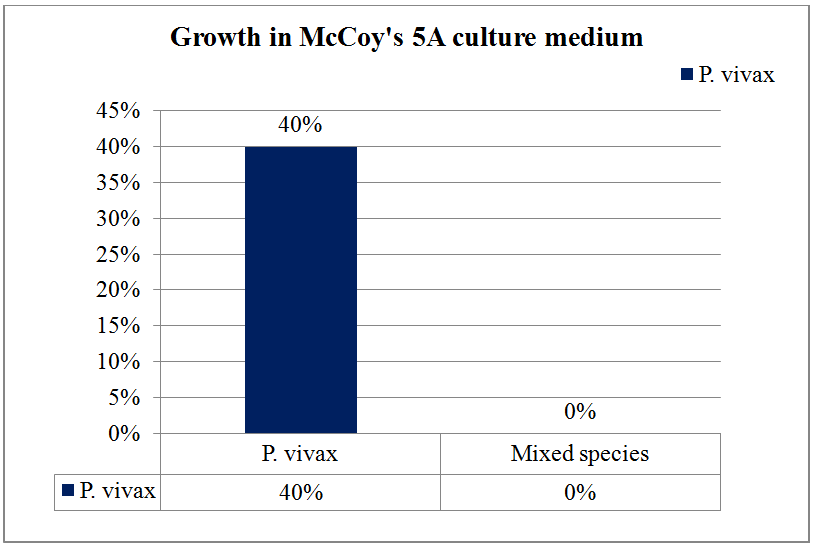 In vitro cultivation of Plasmodium vivax using McCoy’s medium.
In vitro cultivation of Plasmodium vivax using McCoy’s medium.
Singh G, Urhekar AD, Singh R.
Asian J. Med. Pharm. Res., 5(2): 18-21, 2015.; pii:S2252043015000004-5
Abstract
Malaria still poses a threat to the health of residents and travellers in tropical countries, which causes high morbidity and mortality. The present study was undertaken for cultivation of the Plasmodium vivax in McCoy’s 5A medium and observed for their growth. Cultivation of Plasmodium vivax was done according to modified method of Jensen and Trager (1980) in immature red blood cells (reticulocytes) at a 5% haematocrit in McCoy’s 5A medium. Culture medium was sterilized by filtration through a membrane filter of 0.22 μm porosity after addition of supplements. Complete McCoy’s 5A medium was taken in a sterile tissue culture flask and malarial parasites infected blood was added and incubated at 37ºC in 5-10% CO2 incubator. Smears were prepared, stained and examined each day for growth of malarial parasites. Total 22 malaria positive samples were included in this study, out of which 15 were for Plasmodium vivax and 7 were mixed Plasmodium species (Plasmodium vivax and Plasmodium falciparum). Inoculums were incubated at 37°C for 48 hours. After 48 hours of incubation the culture showed 40% growth of Plasmodium vivax and no growth of mixed Plasmodium species. McCoy’s 5A medium supplemented with L-glutamine, HEPES buffer, NaHCO3, hypoxanthine, 0.5% Albumax II and 50μg/ml Gentamicin was useful media for cultivation of Plasmodium vivax.
Keywords: Malaria, Plasmodium vivax, Culture, McCoy’s 5A medium, Filtration, CO2 incubator.
[Full text-PDF] [XML]
Previous issue | Next issue | Archive
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.


